Bird flu could be on the cusp of transmitting between humans − but there are ways to slow down viral evolution
- Written by Ron Barrett, Professor of Anthropology, Macalester College
 Workers who are in frequent contact with potentially sick animals are at high risk of bird flu infection.Costfoto/NurPhoto via Getty Images
Workers who are in frequent contact with potentially sick animals are at high risk of bird flu infection.Costfoto/NurPhoto via Getty ImagesDisease forecasts are like weather forecasts: We cannot predict the finer details of a particular outbreak or a particular storm, but we can often identify when these threats are emerging and prepare accordingly.
The viruses that cause avian influenza are potential threats to global health. Recent animal outbreaks from a subtype called H5N1 have been especially troubling to scientists. Although human infections from H5N1 have been relatively rare, there have been a little more than 900 known cases globally since 2003 – nearly 50% of these cases have been fatal – a mortality rate about 20 times higher than that of the 1918 flu pandemic. If the worst of these rare infections ever became common among people, the results could be devastating.
Approaching potential disease threats from an anthropological perspective, my colleagues and I recently published a book called “Emerging Infections: Three Epidemiological Transitions from Prehistory to the Present” to examine the ways human behaviors have shaped the evolution of infectious diseases, beginning with their first major emergence in the Neolithic period and continuing for 10,000 years to the present day.
Viewed from this deep time perspective, it becomes evident that H5N1 is displaying a common pattern of stepwise invasion from animal to human populations. Like many emerging viruses, H5N1 is making incremental evolutionary changes that could allow it to transmit between people. The periods between these evolutionary steps present opportunities to slow this process and possibly avert a global disaster.
Spillover and viral chatter
When a disease-causing pathogen such as a flu virus is already adapted to infect a particular animal species, it may eventually evolve the ability to infect a new species, such as humans, through a process called spillover.
Spillover is a tricky enterprise. To be successful, the pathogen must have the right set of molecular “keys” compatible with the host’s molecular “locks” so it can break in and out of host cells and hijack their replication machinery. Because these locks often vary between species, the pathogen may have to try many different keys before it can infect an entirely new host species. For instance, the keys a virus successfully uses to infect chickens and ducks may not work on cattle and humans. And because new keys can be made only through random mutation, the odds of obtaining all the right ones are very slim.
Given these evolutionary challenges, it is not surprising that pathogens often get stuck partway into the spillover process. A new variant of the pathogen might be transmissible from an animal only to a person who is either more susceptible due to preexisting illness or more likely to be infected because of extended exposure to the pathogen.
Even then, the pathogen might not be able to break out of its human host and transmit to another person. This is the current situation with H5N1. For the past year, there have been many animal outbreaks in a variety of wild and domestic animals, especially among birds and cattle. But there have also been a small number of human cases, most of which have occurred among poultry and dairy workers who worked closely with large numbers of infected animals.
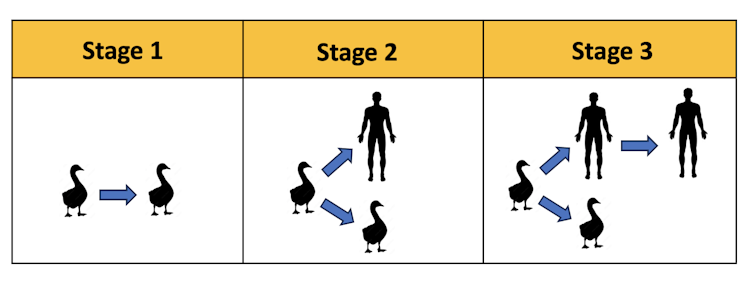 Pathogen transmission can be modeled in three stages. In Stage 1, the pathogen can be transmitted only between nonhuman animals. In stage 2, the pathogen can also be transmitted to humans, but it is not yet adapted for human-to-human transmission. In Stage 3, the pathogen is fully capable of human-to-human transmission.Ron Barrett, CC BY-SA
Pathogen transmission can be modeled in three stages. In Stage 1, the pathogen can be transmitted only between nonhuman animals. In stage 2, the pathogen can also be transmitted to humans, but it is not yet adapted for human-to-human transmission. In Stage 3, the pathogen is fully capable of human-to-human transmission.Ron Barrett, CC BY-SAEpidemiologists call this situation viral chatter: when human infections occur only in small, sporadic outbreaks that appear like the chattering signals of coded radio communications – tiny bursts of unclear information that may add up to a very ominous message. In the case of viral chatter, the message would be a human pandemic.
Sporadic, individual cases of H5N1 among people suggest that human-to-human transmission may likely occur at some point. But even so, no one knows how long or how many steps it would take for this to happen.
Influenza viruses evolve rapidly. This is partly because two or more flu varieties can infect the same host simultaneously, allowing them to reshuffle their genetic material with one another to produce entirely new varieties.
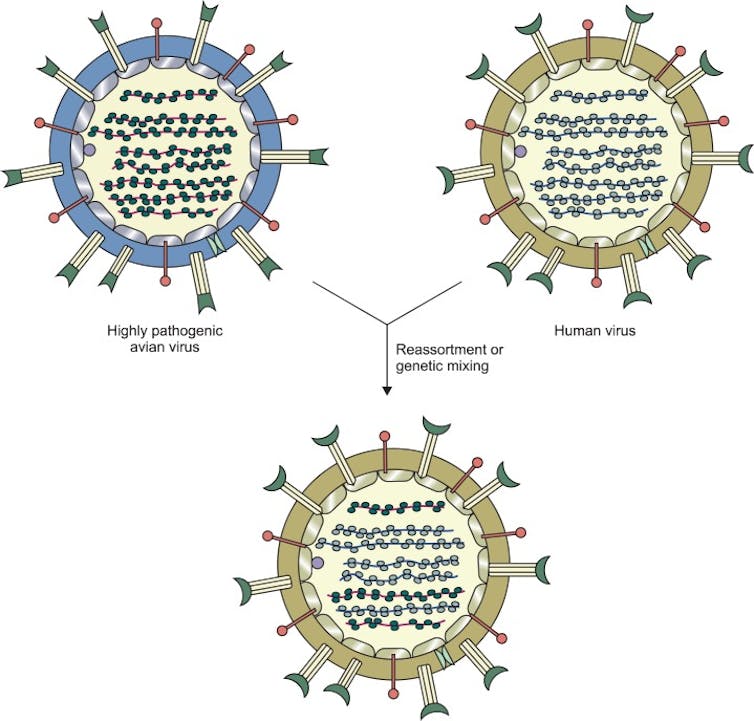 Genetic reshuffling – aka antigenic shift – between a highly pathogenic strain of avian influenza and a strain of human influenza could create a new strain that’s even more infectious among people.Eunsun Yoo/Biomolecules & Therapeutics, CC BY-NC
Genetic reshuffling – aka antigenic shift – between a highly pathogenic strain of avian influenza and a strain of human influenza could create a new strain that’s even more infectious among people.Eunsun Yoo/Biomolecules & Therapeutics, CC BY-NCThese reshuffling events are more likely to occur when there is a diverse range of host species. So it is particularly concerning that H5N1 is known to have infected at least 450 different animal species. It may not be long before the viral chatter gives way to larger human epidemics.
Reshaping the trajectory
The good news is that people can take basic measures to slow down the evolution of H5N1 and potentially reduce the lethality of avian influenza should it ever become a common human infection. But governments and businesses will need to act.
People can start by taking better care of food animals. The total weight of the world’s poultry is greater than all wild bird species combined. So it is not surprising that the geography of most H5N1 outbreaks track more closely with large-scale housing and international transfers of live poultry than with the nesting and migration patterns of wild aquatic birds. Reducing these agricultural practices could help curb the evolution and spread of H5N1.
 Large-scale commercial transport of domesticated animals is associated with the evolution and spread of new influenza varieties.ben/Flickr, CC BY-SA
Large-scale commercial transport of domesticated animals is associated with the evolution and spread of new influenza varieties.ben/Flickr, CC BY-SAPeople can also take better care of themselves. At the individual level, most people can vaccinate against the common, seasonal influenza viruses that circulate every year. At first glance this practice may not seem connected to the emergence of avian influenza. But in addition to preventing seasonal illness, vaccination against common human varieties of the virus will reduce the odds of it mixing with avian varieties and giving them the traits they need for human-to-human transmission.
At the population level, societies can work together to improve nutrition and sanitation in the world’s poorest populations. History has shown that better nutrition increases overall resistance to new infections, and better sanitation reduces how much and how often people are exposed to new pathogens. And in today’s interconnected world, the disease problems of any society will eventually spread to every society.
For more than 10,000 years, human behaviors have shaped the evolutionary trajectories of infectious diseases. Knowing this, people can reshape these trajectories for the better.
Ron Barrett does not work for, consult, own shares in or receive funding from any company or organization that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
Authors: Ron Barrett, Professor of Anthropology, Macalester College

